販売に関して
レクメドは、先天性胆汁酸代謝異常症治療薬 オファコル®カプセル50mg(一般名:コール酸)の日本における製造販売承認を2023年3月27日に取得し、同年6月19日より販売を開始しました。
製品紹介
オファコル®カプセル50㎎(コール酸)
コール酸は、ヒトの肝臓で生合成される一次胆汁酸の主要成分であり、コレステロールを出発物質として肝細胞内のいくつもの酵素反応のステップを経て合成されます。コール酸は消化管からの脂肪吸収を促進し、コレステロールの恒常性の調節、胆汁の分泌を促進する働きがあります。これらの働きは腸肝循環による内因性および外因性の毒物除去に不可欠なものです。
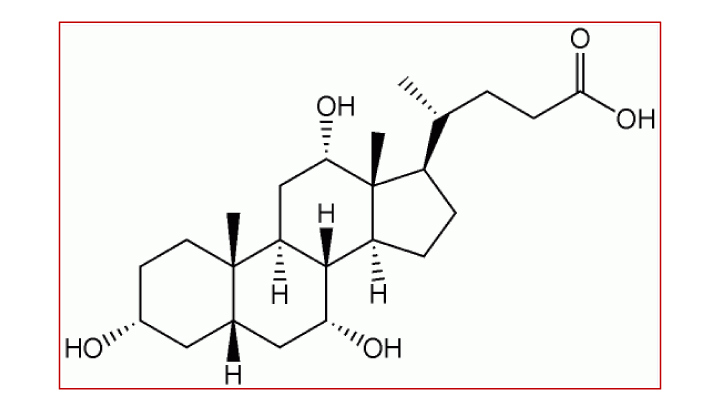
コール酸(CA)
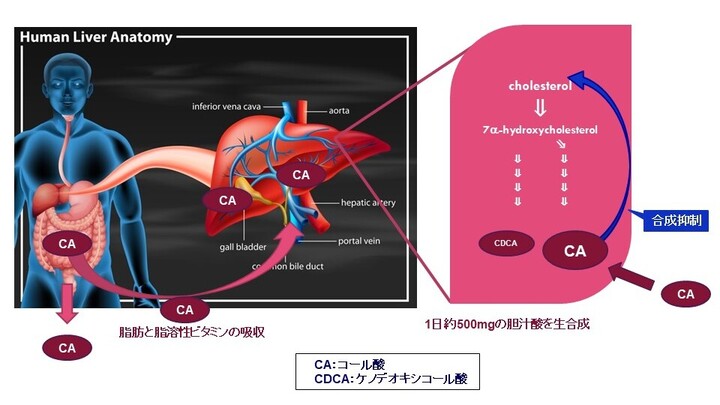
先天性胆汁酸代謝異常症(Inborn Errors of Bile Acid Metabolism: IEBAM)
先天性胆汁酸代謝異常症(単独酵素欠損によるもの)は、肝臓内でのコレステロールから胆汁酸までの生合成経路に関与する酵素のうちいずれか一つの遺伝性欠損を病因とするもので、中間代謝物である胆汁酸前駆物質(異常胆汁酸もしくは胆汁アルコール)の肝細胞内蓄積により肝機能障害を生じる疾患です。重篤な胆汁うっ滞性肝疾患(通常は乳児期に発症)や、小児後期または成人期に発症する進行性の神経系疾患、脂溶性ビタミン欠乏症等を引き起こすことがあります。進行性であり、未治療の場合、肝硬変及び肝不全により死亡に至る恐れがあります。先天性胆汁酸代謝異常症は、小児慢性特定疾病の対象疾病(38)です。患者数は極めて少なく、原因不明の胆汁うっ滞症のおよそ1~2.5 %(注1)、又は5 %程度(注2)存在すると言われています。
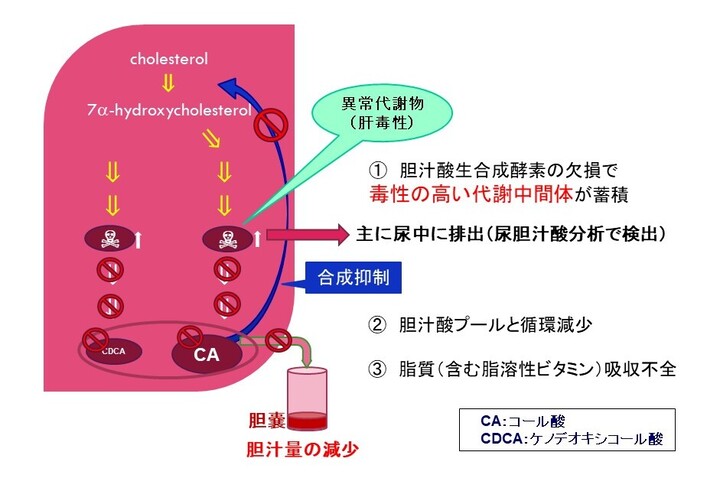
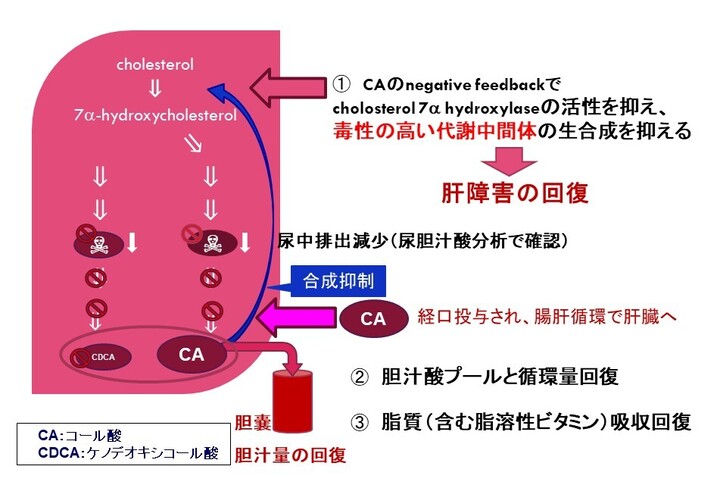
コール酸の作用機序
図に示す通り、これら疾患の患者さんではコール酸が不足しており、コール酸を経口投与することにより、1) 胆汁酸合成を促進する酵素であるコレステロール 7α-ヒドロキシラーゼの発現が抑制されることで、異常な胆汁酸の産生を抑制します (Setchell & O’Connell, 2007)。さらに、2) コール酸の経口投与は、胆汁の流れを賦活化し、異常な胆汁酸やビリルビンを含む毒性物質の肝クリアランスを促進することにより、生化学的及び組織学的異常を回復させ、肝機能を改善します。また、3) 脂溶性ビタミンと脂肪の吸収を促進することにより、成長阻害を改善します。
コール酸補充療法
コール酸は、欧米では既に医薬品として販売されており、先天性胆汁酸代謝異常症に対して有効性及び安全性が確立された治療法として標準的治療法とされています。(注3)
一方日本では、先天性胆汁酸代謝異常症に対して適応を有する薬剤がこれまで無く、医療上の必要性の高い未承認薬として開発企業の募集が行われ、レクメドがフランスのLaboratoires CTRS社(現THERAVIA社)との共同開発にて国内第3相試験を実施し、新薬製造販売承認を取得しました。本剤は希少疾病用医薬品(指定番号(R2薬)第481号)にも指定されております。
注 1 : Setchellet et al., Liver Disease in Children. Mosby, St Louis: pp81-104, 1994
注 2 : 木村 他, 日児誌, 104: 686-7, 2000
注 3 : Gonzales et al., Orphanet Journal of Rare Disease, 13: 190, 2018